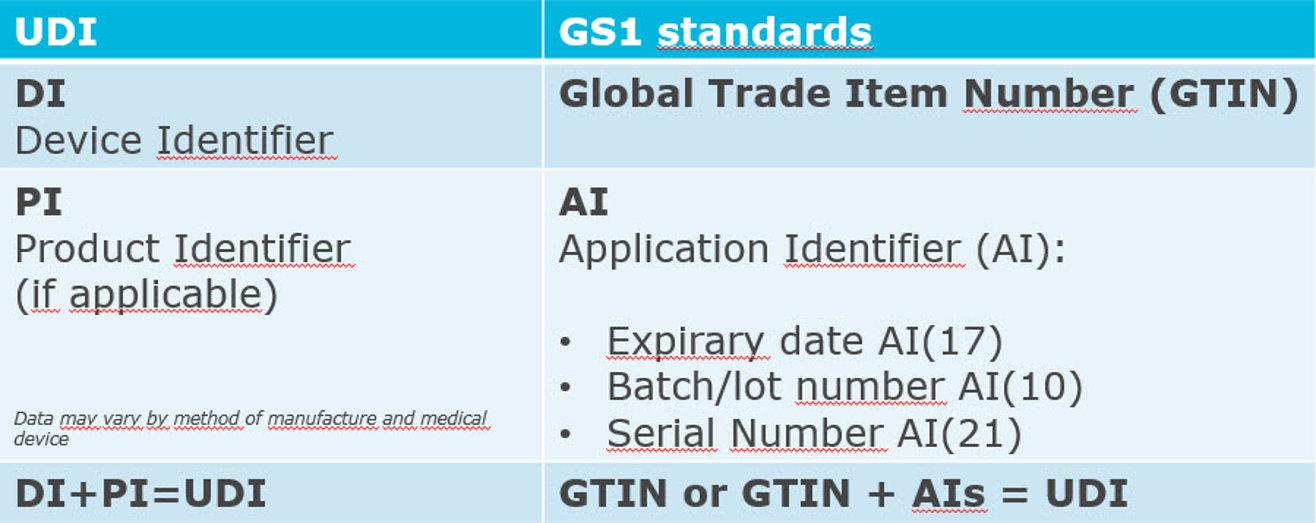
A UDI is a unique numeric or alphanumeric code and consists of two parts:
A Device Identifier (DI): mandatory part for the unique identification of the medical device or IVD. A Production Identifier (PI): variable part with manufacturing data.
Creating a UDI
Order GS1 article codes/GTINs
Assign a GS1 article code to each version or model in order to uniquely identify the product. Use the GTIN-13 for this purpose. Because the healthcare barcodes (GS1-128 and GS1 Data Matrix) have 14 positions, you manually add a leading zero. Or you can use indicator digits to identify packaging levels, in which case you also get 14 positions.
Creating Basic UDI-DI (GMN)
Creating the Basic UDI-DI/GMN is to assign the medical device to a product family. To determine the basic details of a GMN, the GS1 code package is required.
Order GS1 article codes/GTINs
Assign a GS1 article code to each version or model to uniquely identify the product. And assign the DI to a product family (GMN).
To do this, use the GTIN-13. Because the GTIN in healthcare barcodes (GS1-128 and GS1 DataMatrix) has 14 positions, manually add a leading zero. Or you use indicator digits to identify packaging levels to get to 14 positions as well. Further explanation in the document: Guidelines for assigning GTINs
Adding manufacturing data
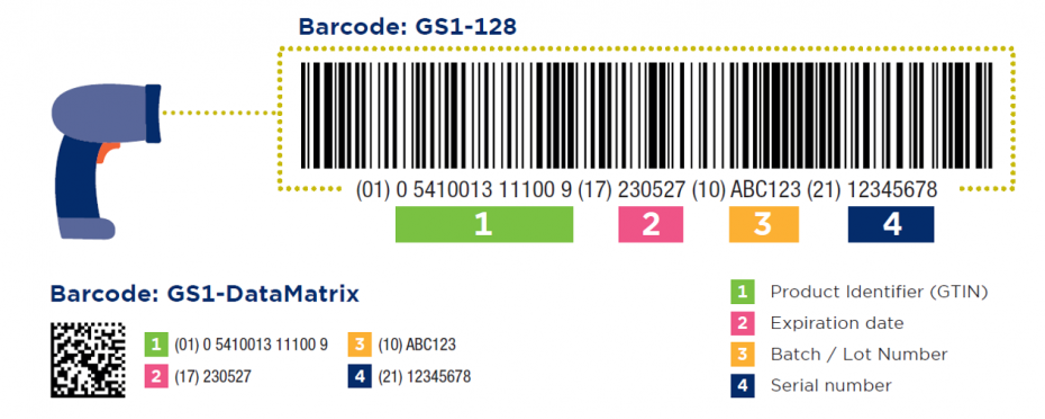
Depending on the requirements of the legislation; add production date, expiry date, batch/lot number or a serial number. Use Application Identifiers (AIs) for this purpose. In the case of software, a software version can be added.
Place UDI in text form and as a symbol on the product
In healthcare, the GS1-128 and the GS1 DataMatrix are the two permitted barcodes worldwide. The manufacturer of the medical device chooses which barcode needs to be on the product.
Medical devices for retail sale
For medical devices that are exclusively sold in retail, it is not necessary to include the production data in the barcode. Therefore, an EAN-13 barcode is sufficient. However, the production data must still be printed legibly on the packaging.
Help with UDI
Partners can help you create and print a UDI in barcode form. GS1 also works with partners who can help with consultancy or product data sharing.
EU UDI Helpdesk
Support vanuit de Europese Commissie.
Webinar
Webinar given by GS1 about UDI en Basic UDI-DI
